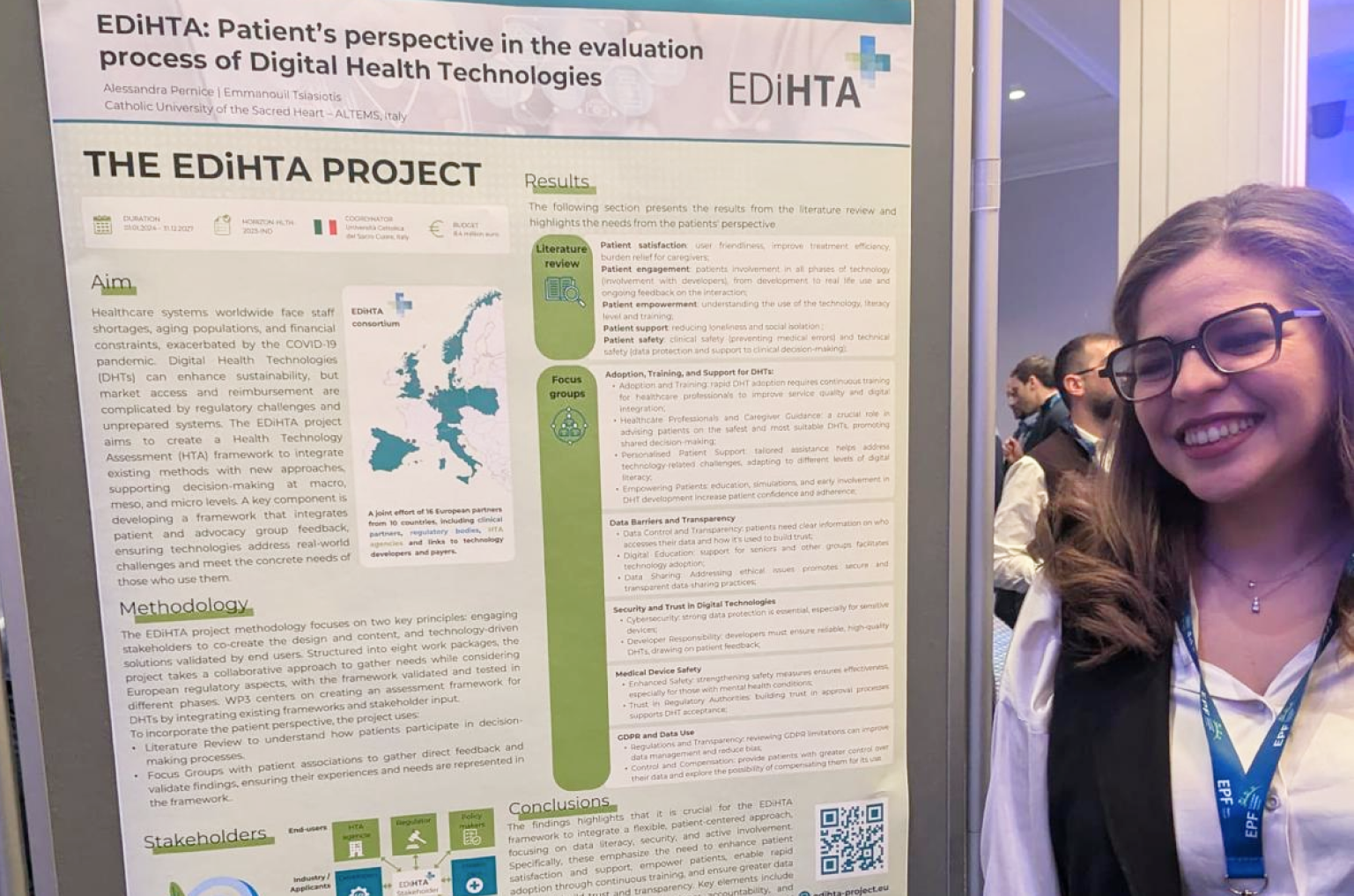
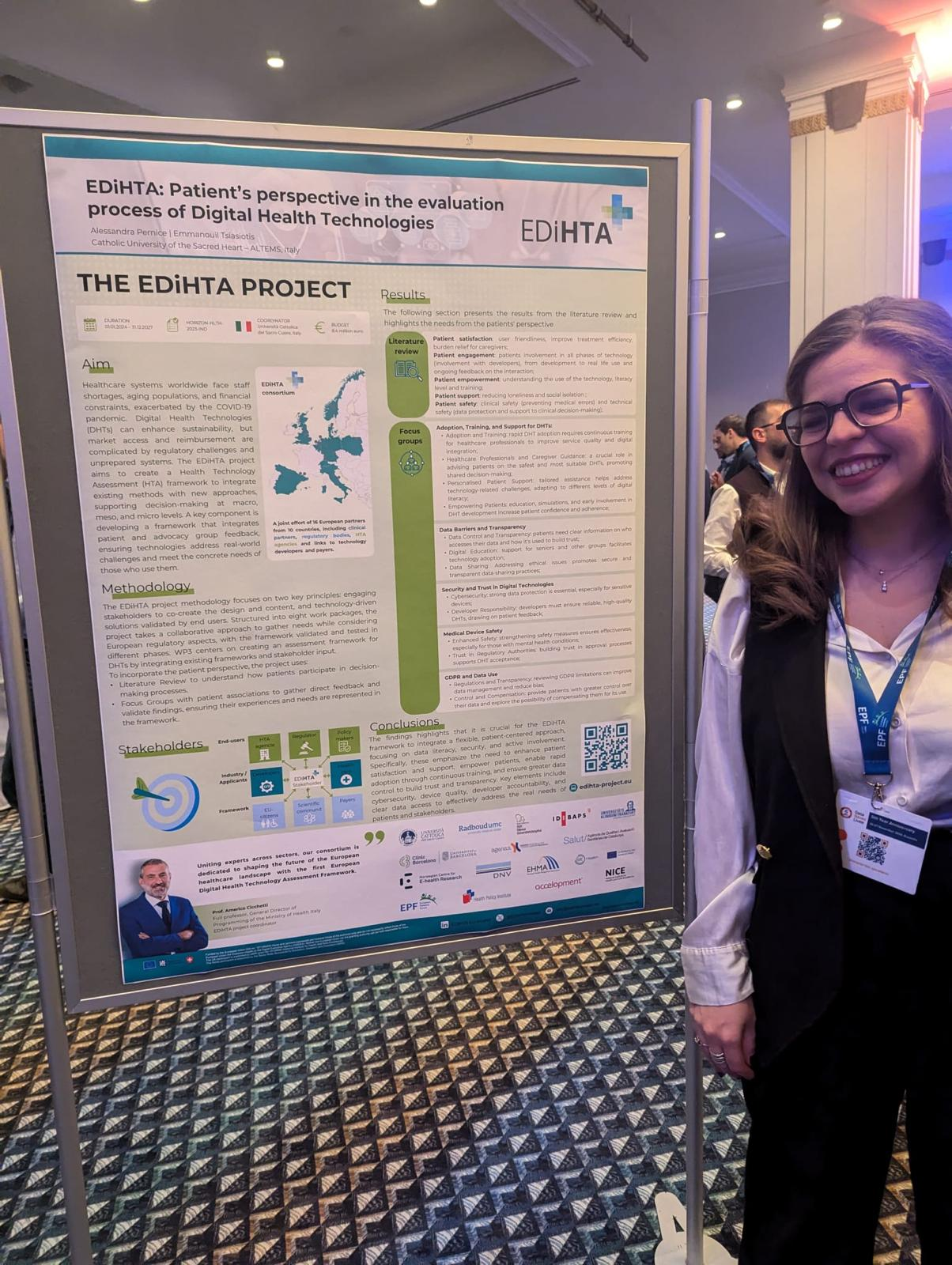
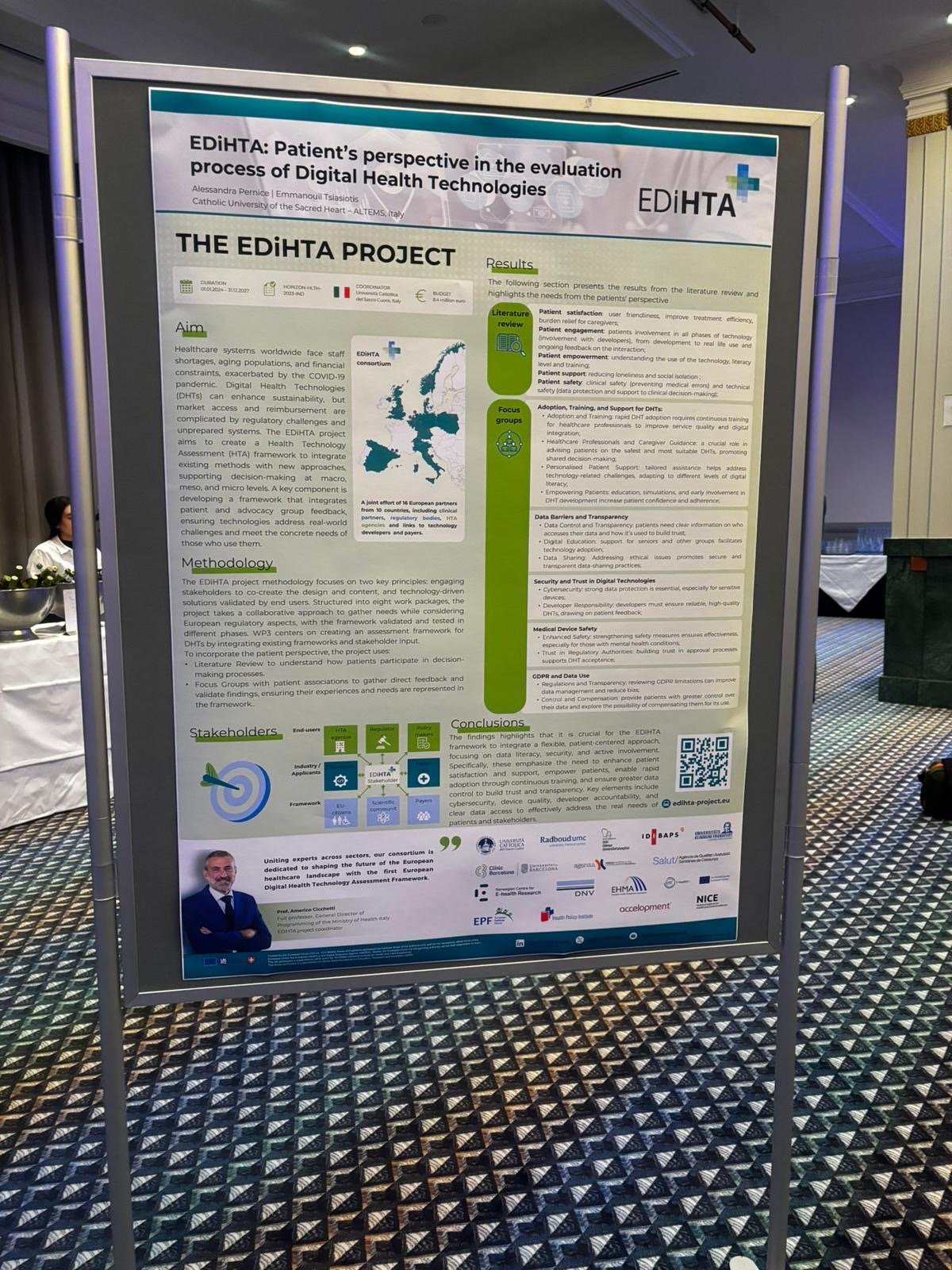
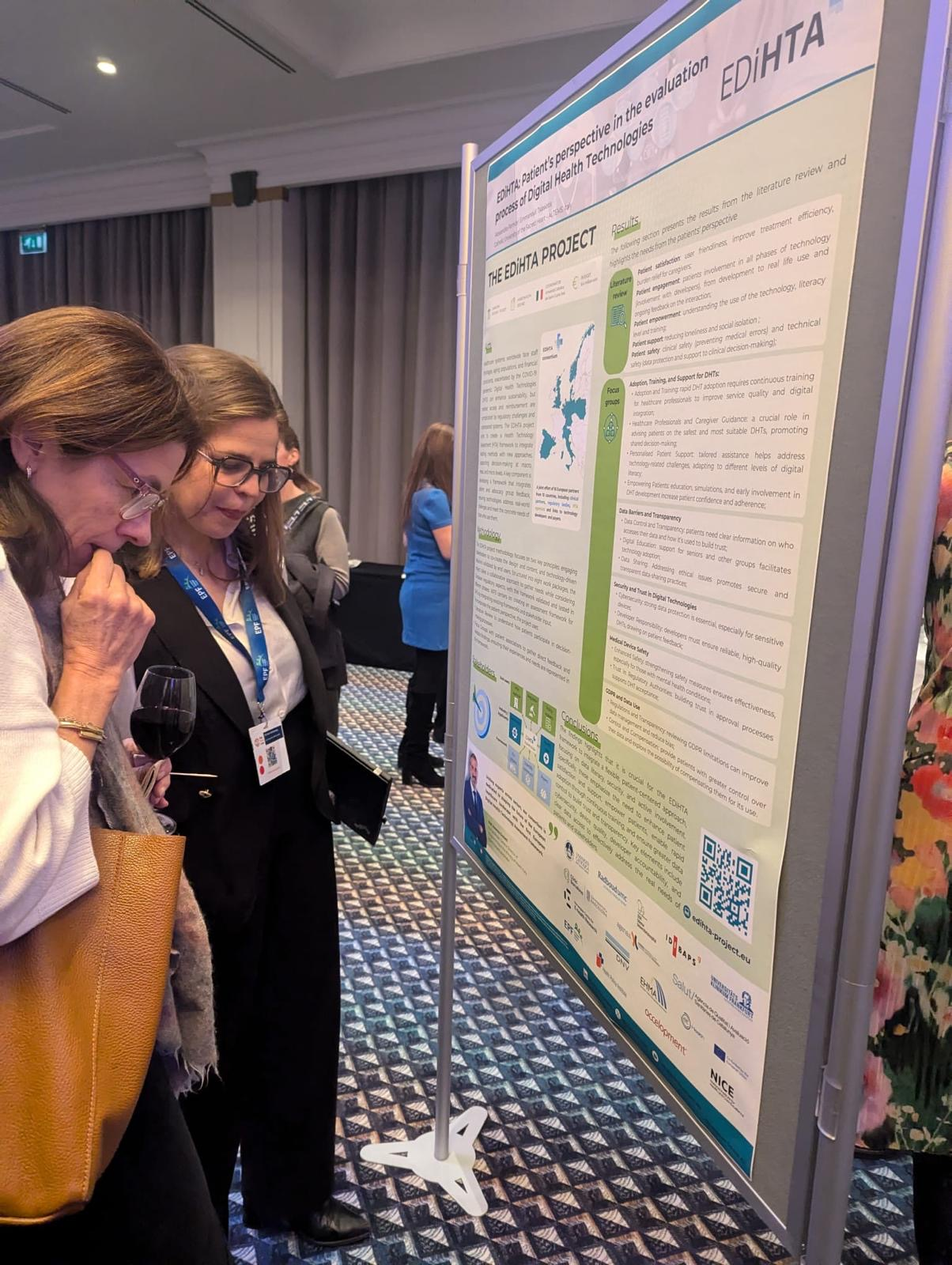
We are excited to announce that our partner from AQuAS, Joan Segur-Ferrer, will be presenting the EDiHTA project at the XIII Meeting of the Working Group on Imaging in Dermatology, taking place on March 14, 2025, in Barcelona. Joan’s presentation, titled “EDiHTA: The First European Framework for Digital Health Technology Assessment, Co-Created by Stakeholders Across the European Health Ecosystem”, is scheduled for Friday, March 14, from 15:30 to 15:45, during the Plenary Session III of the event.
The EDiHTA project plays a crucial role in shaping the future of digital health technology assessment (HTA) by bringing together key stakeholders from across Europe to establish a unified framework. Joan’s talk will provide valuable insights into the project’s objectives, methodologies, and its impact on the evolving landscape of health technology evaluation.
The XIII Meeting of the Working Group on Imaging in Dermatology, organised by the Spanish Academy of Dermatology and Venereology (AEDV) and the Spanish Group of e-Dermatology and Imaging (GEDEI), gathers experts in teledermatology, AI in dermatology, ultrasound, confocal microscopy, and dermatoscopy to discuss the latest advancements in the field.
Stay tuned for updates following Joan’s presentation!