THIS WEBSITE USES COOKIES
We use cookies to personalise content, to provide social media features, and to analyse our traffic. By choosing 'allow all cookies', you consent to our cookies.
To find out more, read our privacy policy and cookie policy.
The EDiHTA framework is poised to create a new paradigm for the assessment and adoption of digital health technologies in Europe. Through collaboration, innovation, and a patient-centered approach, the project aims to accelerate the integration of transformative digital tools into healthcare systems, ensuring that Europe remains at the forefront of digital health innovation. By ensuring patient involvement and stakeholder collaboration at every stage, EDiHTA promises to be a game-changer in how digital health tools are evaluated and adopted across the EU.
A key aspect of the EDiHTA framework is the active involvement of patients, a group often underrepresented in the development of health technologies. The European Patients’ Forum (EPF), a core patient advocacy partner in the project, is working to ensure that patient perspectives and needs are incorporated into the development of the EDiHTA framework. EDiHTA has established a Patient Advisory Group (PAG), composed of patients and patients’ representatives with direct experience in using digital health tools. Their input is critical in defining relevant endpoints, assessing the usability of Diital Health Technologies (DHTs), and ensuring that patient-centric values are integrated into the evaluation process. The PAG consists of fifteen members representing a variety of countries (Germany, Greece, Estonia, Finland, Slovakia, Spain, Malta, Italy and the UK) and disease areas, including diabetes, cancer, rheumatic, cardiovascular and rare diseases. Their diverse backgrounds and experiences contribute to a comprehensive understanding of patient needs and priorities. The PAG members of EDiHTA have been active in patient organisations for several years and have also participated in various EU-funded projects. The PAG will play a pivotal advisory role across the project’s various activities and contribute to dissemination and communication efforts within their respective communities.
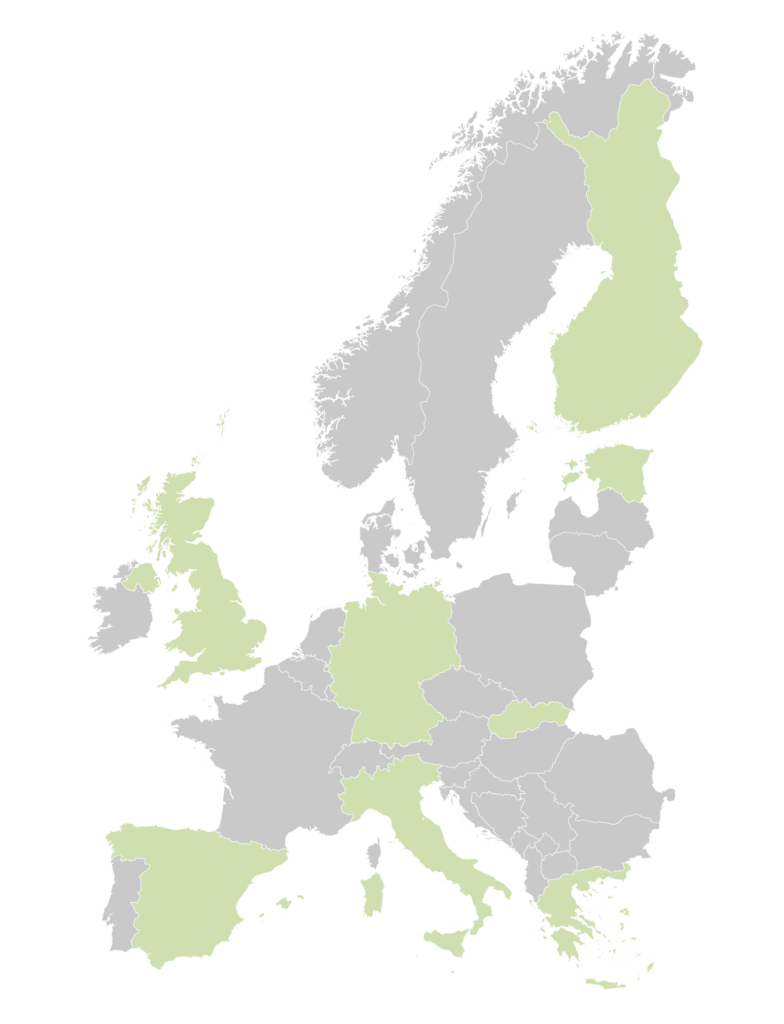
Germany
UK
Italy
Spain
Finland
Slovakia
Greece
Malta
Estonia
Through focus groups and other engagement activities, patients provide insights on the effectiveness, accessibility, and relevance of DHTs such as Artificial Intelligence, Telemedicine, Medical Devices and Robotics in real-world settings. This participatory approach is not only essential for improving the design of health technologies but also for fostering trust, transparency, and accountability in the HTA process. By involving patients from the outset, EDiHTA aims to produce outcomes that align with real-world healthcare needs and improve the overall quality of digital health intervention.
Artificial Intelligence
Telemedicine
Medical Devices
Robotics
To accomplish this, the PAG will contribute by participating in two focus group meetings, engaging in stakeholder meetings, monitoring project progress, providing feedback as required, and attending project meetings, when needed. The PAG’s input will be incorporated into the project through various mechanisms, ensuring that the patient experience remains a key focus in EDiHTA’s work



Prof. Americo Cicchetti
EDiHTA project coordinator
Stay informed about latest EDiHTA results, conferences, and milestones.