THIS WEBSITE USES COOKIES
We use cookies to personalise content, to provide social media features, and to analyse our traffic. By choosing 'allow all cookies', you consent to our cookies.
To find out more, read our privacy policy and cookie policy.
HTA processes differ significantly across countries, shaped by their healthcare systems’ structure, with national or regional implementations sometimes causing duplication of clinical benefit assessments, particularly in decentralised systems. Most HTA recommendations are non-binding, but their impact on coverage decisions varies; in some countries non-binding recommendations are de facto mandatory for reimbursement. EU cooperation could streamline HTA activities, reduce duplication of clinical evidence assessments, and harmonise methodologies, especially for medical devices, where evaluation processes are fragmented and less advanced. Stakeholder engagement, including patients and healthcare experts, varies widely, influencing transparency and uptake of HTA outcomes.
HTA agencies are integral to the EDiHTA project, contributing their expertise from the outset to shape a scientifically robust and practically applicable framework. Their active involvement in pilot testing and validation ensures the framework remains relevant to the evolving digital health landscape while meeting high assessment standards.
HTA agencies will benefit from EDiHTA by gaining enhanced capabilities to evaluate digital health technologies with a harmonised, evidence-based framework that promotes consistency across Europe. The project supports capacity building through training, knowledge sharing, and networking opportunities, enabling agencies to stay at the forefront of digital health assessment. Additionally, their involvement helps shape future guidelines and regulatory standards, influencing digital health policies while ensuring patient care, cost-effectiveness, and innovation are prioritised.
EDiHTA is dedicated to equipping HTA agencies with the tools and insights needed to effectively evaluate Digital Health Technologies. Collaborating alongside regulatory bodies, clinicians, payers, researchers, and patient representatives, HTA agencies help foster a comprehensive and multidisciplinary approach to evaluating DHTs. Furthermore, as key players in healthcare systems, HTA agencies have the power to drive innovation while safeguarding patient safety and advancing sustainable healthcare solutions.
Norwegian Medicines Agency (NMA)
Finnish Coordinating Center for Health Technology Assessment
State Health Care Accreditation Agency
Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT, The Agency for Health Technology Assessment and Tariff System)
National Health Care Institute (ZIN)
Breadcrumb Home News Luxembourg Agency for Medicines and Health Products
French National Authority for Health
Health Information and Quality Authority (HIQA)
National Institute of Pharmacy and Nutrition (NIPN)
National Agency for Medicines and Medical Devices of Romania
National Council on Prices and Reimbursement of Medicinal Products
Minstry for Health and active Ageing (MFH), Directorate for Pharmaceutical Affairs (DPH)
Ministry of Health
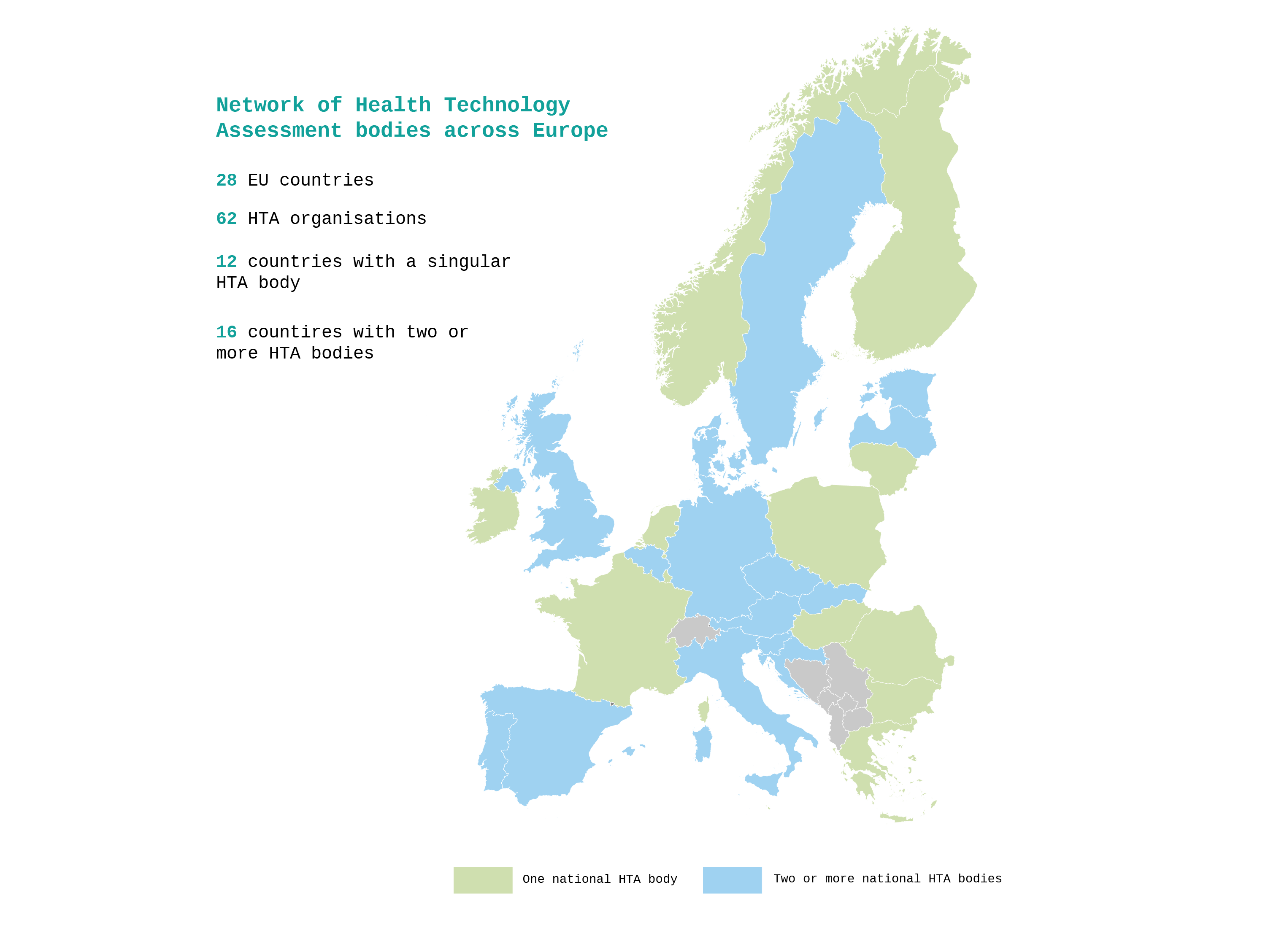
A national HTA organisation/body is a legal entity having a clearly defined, often formally supported through legislative acts, role in the national HTA production process to inform decision-making on reimbursement, pricing and/or provision of health technologies in the country.
HTA processes differ significantly across countries, shaped by their healthcare systems’ structure, with national or regional implementations sometimes causing duplication of clinical benefit assessments, particularly in decentralised systems. Most HTA recommendations are non-binding, but their impact on coverage decisions varies; in some countries non-binding recommendations are de facto mandatory for reimbursement. EU cooperation could streamline HTA activities, reduce duplication of clinical evidence assessments, and harmonise methodologies, especially for medical devices, where evaluation processes are fragmented and less advanced. Stakeholder engagement, including patients and healthcare experts, varies widely, influencing transparency and uptake of HTA outcomes.
HTA agencies are integral to the EDiHTA project, contributing their expertise from the outset to shape a scientifically robust and practically applicable framework. Their active involvement in pilot testing and validation ensures the framework remains relevant to the evolving digital health landscape while meeting high assessment standards.
Your input matters: Join us in our mission to shape the future of the European HTA landscape.
Do you represent an HTA agency and you want to collaborate with us? Get in touch!

Prof. Americo Cicchetti
EDiHTA project coordinator
Stay informed about latest EDiHTA results, conferences, and milestones.