THIS WEBSITE USES COOKIES
We use cookies to personalise content, to provide social media features, and to analyse our traffic. By choosing 'allow all cookies', you consent to our cookies.
To find out more, read our privacy policy and cookie policy.
Digital Health technologies implementation varies across the different health systems in Europe, whilst the specific characteristics of different markets result in an increasing level of complexity for the developers. Furthermore, current national market access schemes and HTA processes are created without considering the need for guidance for the developers that will apply, therefore resulting in an inefficient evaluation process of the developed technologies that takes a lot of time and sometimes fails to reach a successful implementation of the technology in the market of interest.
Industry stakeholders are in need of more guidance regarding the evidence needed to demonstrate the added value of digital health technologies; therefore, the EDiHTA consortium has set out to involve technology developers throughout the entire process of framework development, from conceptualisation to piloting. From the first year of the project, we have successfully involved representatives of start-ups, SMEs and multinational companies that are part of 9 different European markets (Austria, Belgium, Finland, France, Germany, Italy, Poland, Ukraine, UK) whilst collaborating closely with MedTech Europe.
In 2024, we identified the needs and requirements of this specific stakeholder group regarding current challenges of market access, reimbursement and HTA, whilst also imagining the future of the DHTs market in Europe and the possible role of EDiHTA in supporting a European market for these technologies. Developers and manufacturers will also be involved in the validating phase of the EDiHTA framework in 2026, more specifically during an evaluation workshop and the piloting phase where three technologies will be selected through an open call to test the digital platform of the EDiHTA framework.
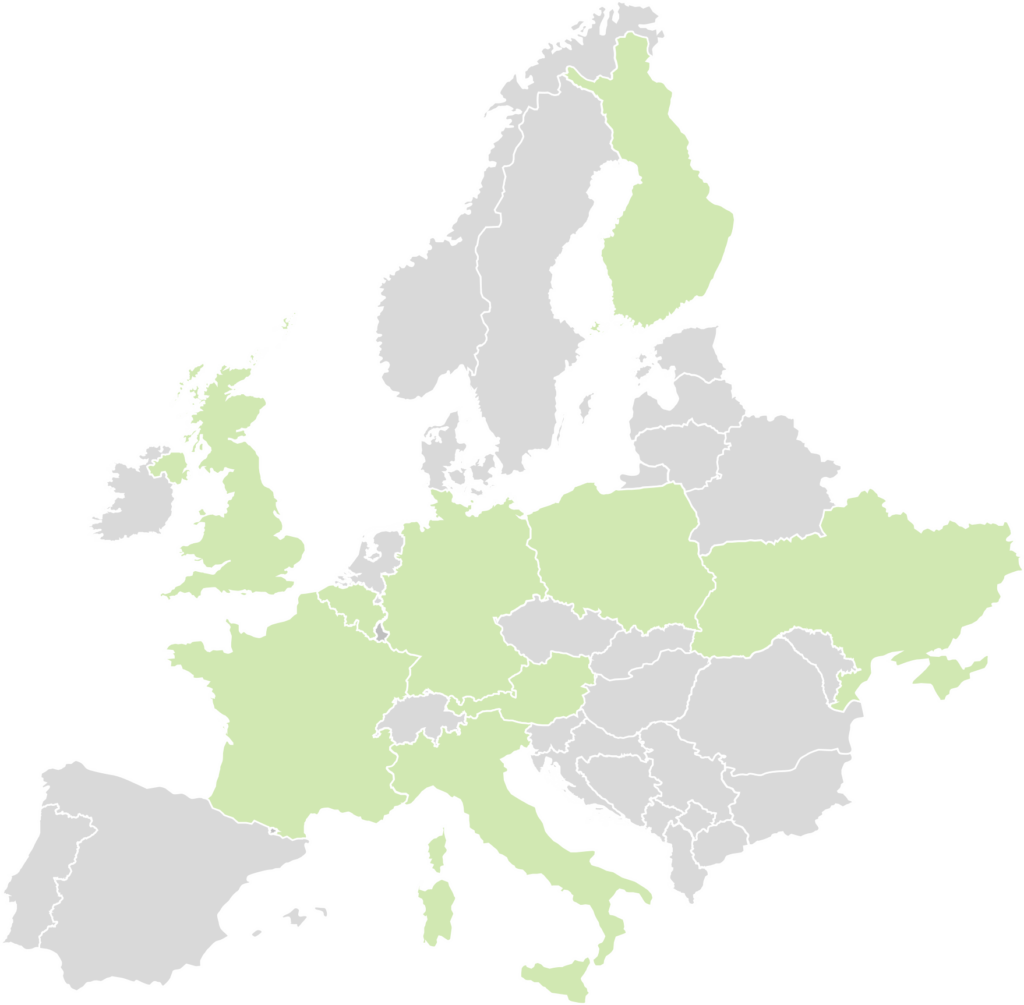
France
Germany
Italy
Austria
Finland
Ukraine
Poland
Belgium
UK

EIT Health supports the EDiHTA project in interfacing with industry stakeholders, including start-ups, SMEs and large corporations from different industry verticals (MedTech, Digital Health, etc.) that are developing digital health technologies and solutions. In EDiHTA, EIT Health has worked with partners to facilitate surveys and focus groups to map out the challenges, needs and best-practices of digital health technology developers (including mobile apps, telemedicine platforms, AI solutions, etc) in relation to health technology assessment processes. Additionally, EIT Health will be engaging in piloting activities to validate the EDiHTA framework and corresponding digital platform and tools, supporting the selection of three digital technology use cases through an open call to the ecosystem Finally, EIT Health supports the exploitation of the future EDiHTA framework and platform through identifying and catering for the education and training needs of industry stakeholders.
Your input matters: Join us in our mission to shape the future of the European HTA landscape.
Are you a technology developer and you want to collaborate with us? Get in touch!

Prof. Americo Cicchetti
EDiHTA project coordinator
Stay informed about latest EDiHTA results, conferences, and milestones.